RESEARCH BACKGROUND
We now have over 20 years of field and laboratory experience with the enzyme formula used in XBEE Enzyme Fuel Technology.
We have conducted many laboratory studies on the effects of this unique product. Our independent laboratory testing has focused primarily on XBEE‘s efficacy in purifying fuel and improving combustion, as well as engine and environmental safety. These tests, as well as field studies conducted by customers and OEM’s, give us the empirical proof that XBEE is indeed an extraordinary fuel treatment.
A great deal of academic research on the mechanistic behaviour of enzymes on hydrocarbon substrates was conducted in the late 1970’s and 1980’s, primarily for oil-spill remediation and for bio-desulfurization of crude oil. In fact, XBEE can trace its roots to the industrial sludge treatment and water purification industries.
MECHANISMS OF ENZYME CATALYSIS
There is a significant body of scientific theory on the mechanistic function of enzymes within biological cells, as well as many decades of research on the extracellular enzymes, function in industrial applications such as enhancing petroleum recovery in wells and soil remediation. The mechanistic function of enzymes in non-polar, organic solvents such as fuel is less studied, but several research papers over the past ten years have described how enzymes suspended in organic solvents can catalyze novel reactions that are impossible in aqueous solutions.
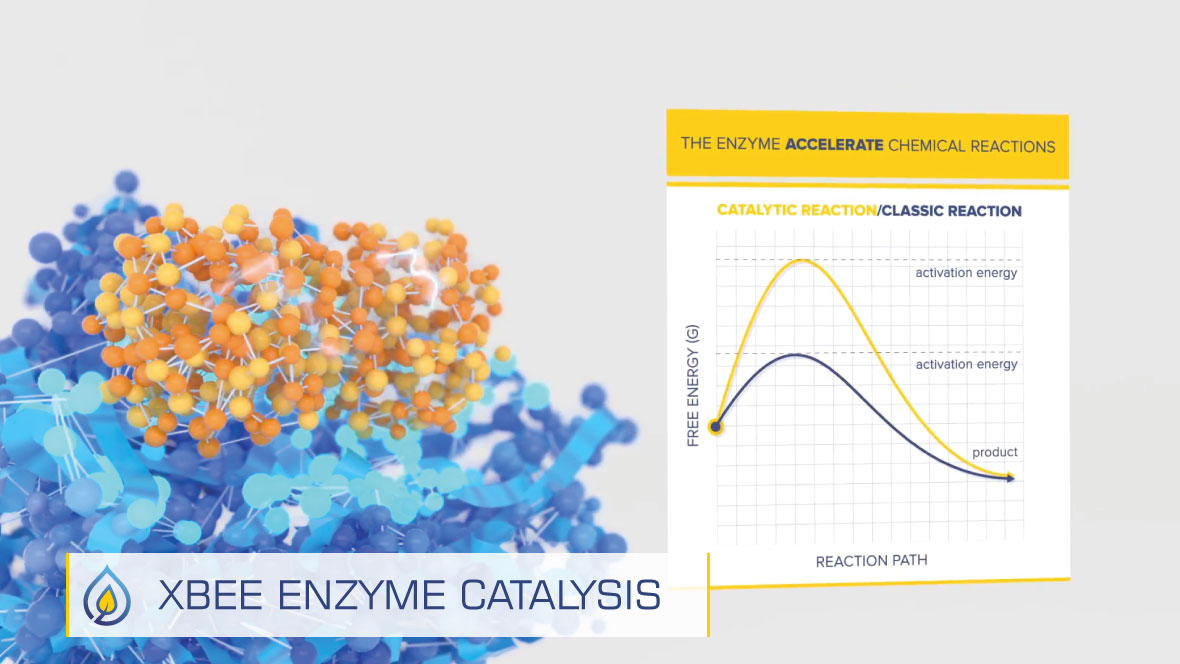
Enzymes are catalysts that lower the activation energy of a reaction by providing a pathway(s) with lower activation energy than in a nonenzymatic reaction. For many years, a simple “lock and key” theory was used to describe enzyme-substrate specificity and function.
This simple model can still be used, but with a major stipulation: the substrate is not the “key”. The key is the “Transition State”. Generally, enzymes collide with substrates as they diffuse through the fuel, although some may be further attracted to substrates (target molecules) by electrostatic charges.
They contact their target molecules and form Enzyme-Substrate complexes, called the “ES”. Enzymes alter the electrical charges on substrates, temporarily binding to them. Between the starting point; a substrate binding to an ES and the release of the product, there is a temporary state called a transition state, which can be described as a high-energy structure somewhere between the reactant and product. After the ES is formed, the enzyme distorts the substrate, forcing it to the transition state. It is the transition state, rather than substrate, that is the key.
The substrate reacts only transiently with the enzyme. After catalysis, or “conversion”, the product is released and the enzyme is free to perform more reactions. Some enzymes, under the right conditions, can perform catalysis as fast as the enzyme can diffuse into the solution. Reaction rate acceleration can be as high as 1,017 faster than non-enzymatic reactions. One recent researcher has identified some enzymes could be as much as 1,021 faster. Enzymes can perform cleavage reactions, as previously described with PAH molecules. Hydrocarbons with covalent bonds (where pairs of electrons are shared by two atoms) are separated by the enzyme, with either one atom retaining both electrons, or both atoms each retaining just one electron. The substrate, once a large, difficult to burn molecule, is converted to two smaller molecules. To the engine, this means the fuel is more easily evaporated during injection, and can be ignited easier and react with more oxygen to burn more completely.
The enzyme’s amazing speed at catalysis stems from binding modes. The binding forces behind ES complexes are charge-charge interactions, hydrogen bonds, hydrophobic interactions, and van der Waals forces. Charge-charge interactions are stronger in non-polar environments such as organic solvents than in water. It is believed that XBEE‘s extraordinary speed, efficacy, and longevity are derived from this phenomenon.
The substrate affinity and speed of catalysis are dependent upon the three-dimensional structure of the enzyme. Enzymes evolved to function in aqueous solutions and the position of individual water molecules within the enzyme’s active sites are critical to both substrate sensitivity as well as the rate of catalysis. A monolayer, perhaps less than 500 water molecules, are always present, permanently attached to the surface of the enzyme. It is believed that when suspended in a non-polar, organic solvent, the hydrophobic force of the solvent pushes on the water molecules, in effect, forming a cage that holds the enzyme in its three-dimensional shape. This allows the enzyme to function over extraordinarily long periods of time, as well as improve its thermal stability.
Several thousand enzymes have been identified in animals. While most textbooks discuss the highly specialized actions of enzymes, known as “substrate specificity”, this is a derivative of enzyme research having been financed primarily for human or animal biological studies. Plant enzymology is less studied. Plants evolved roughly five hundred million years before animals, at a time when the prehistoric soil was relatively barren of organic matter.
Plants typically produce only a few dozen different enzymes, some of which evolved to catalyze multiple substrates in order to secure nutrients critical to their growth. This phenomenon is known as “enzyme promiscuity”.
Over two dozen plant enzymes are used in XBEE which provides a wide range substrate reactivity.
As previously noted, XBEE has surfactant properties. Standardized ASTM testing has demonstrated this phenomenon in petroleum fuels. Recently, other researchers working with enzymes within the same class as those used in XBEE have demonstrated that enzymes can work in concert with each other, resulting in a surfactant affect that is collectively stronger than the individual enzymes. These enzymes break down sludge and disperse microscopic drops of water during fuel agitation. An enzymatic bio-surfactant differs from the chemical surfactants used in detergents in that they never become permanently bonded to the substrate. Enzyme breaks the electrical bonds holding the sludge to the fuel tank walls and helps dissolve the sludge into smaller complexes, as would a detergent. The enzyme then releases from the dissolved sludge, or “products”, and is free to act over and over, almost indefinitely. Chemical surfactants act only once, and become bonded to the product. Therefore they have a finite reach and lifespan in fuel. Far fewer enzyme molecules are required to do the same amount of cleaning work as many times their amount of chemical surfactants. This eliminates the potential precipitation of chemicals that occasionally plagues conventional fuel additives if overdosed.
EFFECT ON EMISSIONS
As mentioned previously, XBEE‘s cleaving action reduces the size of the larger aromatic hydrocarbons that are precursors to soot. Aromatics have an opposite relationship to cetane index and number, and fuels that have high aromatics are typically lower in cetane ratings compared to fuels with low aromatics. XBEE can significantly increase cetane ratings in fuels by reducing these larger aromatics. This function helps improve cold-starts, as well as generally improves combustion, and reduces the most toxic exhaust fractions; the PAH molecules.
Additionally, XBEE‘s effect on reducing surface tension results in smaller fuel droplets and faster evaporation. When combined, these two effects result in hyper-oxygenation of the fuel as it is injected into the combustion chamber. This leads to better and more complete combustion. Emissions tests demonstrate this effect through a reduction in CO and HC, including the toxic PAH fractions. Fewer soot results in a reduced rate of soot contamination in the lube oil, as well as preventing hardened carbon deposits from forming in the combustion chambers.
As the concussion forces in the engine dislodge old carbon deposits, combined with the action of the XBEE injector cleaner in preventing the formation of carbon deposits, engines are cleaned over a period of time. Endoscopic studies of commercial marine engines by OEMs confirm these benefits as a result of the improved combustion of XBEE. Inside the combustion chamber, nitrogen in the air is oxidized to NOx. Higher combustion chamber temperatures result in a higher NOx level. By improving the vaporization rate and ignition characteristics, XBEE gives the fuel charge more time to burn completely. This reduces or eliminates the amount of still-burning fuel that comes in contact with the cylinder walls or is pushed past the exhaust valves, overheating them. This tends to reduce NOx, even as CO, HC, and soot particles are reduced.